4d 5 5s 1 Electron Dot Model. Determine the number of 3d electrons in Cr.

Molybdenum Mo Electron Configuration And Orbital Diagram
Which of the following is the electron configuration for Mo.
. 1The number of electrons in 4d subshell of Mo 5. A 1 B 4 C 3 D 5 E 2. What is the Maximum number of electrons into 4d orbital.
Determine the number of 3d electrons in Cr. 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 5. Submit My Answers Give Up Part C Determine the number of 4d electrons in Mo Express.
Use the periodic table to determine each quantity. Find the slope of the line graphed below. The electronic configuration of Mo is Kr 5s1 4d5.
4dy 4dx 4dz 4dz2 4dx2-y2Each of these can fit 2 electrons. Electrons per Energy Level. For Mo it is in the 5th row of the periodic table Mo.
This problem has been solved. The number of 4d electrons in Y. Mo3 Kr 4d3.
Number of Neutrons most commonstable nuclide. Name an element in the fourth period row of the periodic table with two 4p electrons. Submit My Answers Give Up Part B Determine the number of 3d electrons in Cr Express your answer as an integer.
The number of 3s electrons in Mg. Determine the number of 6p electrons in Po. 4d 2 5s 2.
It shows the order of electron entry for the building up process. Determine the number of 4d electrons in Mo. So 1 electron is promoted from the 5S to the 4d Same exception occurs with the d orbital has 9 electrons.
An S is promoted to fill the d. The number of 3d electrons in Cr. Expert solutions for 11How many 4d electrons does the Mo atom have.
Determine the number of 4d electrons in Mo. Chemical Properties of Molybdenum. 4d 4 5s 1.
Determine the number of 3d electrons in Cr. It is NOT Kr 5s2 4d4. 11 rows Electron configurations filling orbitals and valence electrons of 4d elements.
How many 4d electrons does a ground-state Mo atom have. Name an element in the fifth period row of the periodic table with five valence electrons. 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 2 5s 2.
There are 5 total 4 d orbitals. Find step-by-step Chemistry solutions and your answer to the following textbook question. This rule is known as.
This is an exception to the filling rule because sometimes half filled d orbitals have lower energy than filled s. The number of 6p electrons in Pb. For Mo 3 ionization of transition metals always starts from the valence level the highest principle Quantum Number 5 then moves into the 4d level.
Express your answer as an integer. Number of Electrons with no charge. Provide an appropriate responseMissouri charges a 6 sales tax and Springfield MO charges an additional 655 city sales tax.
How many 4d electrons does a ground-state Mo atom have. Determine the number of 4d electrons in Mo Determine the number of 6pp electrons in Po Given an int variable k that has already been declared write some code that uses a for loop to print a single line consisting of 97 asterisks. Determine the number of 6pp electrons in Po.
The ground-state electron configuration of Cr Mo and Ag are exceptions to the Aufbau principle. 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 4 5s 1. Atomic Number Symbol Name Electron Configuration.
Show transcribed image text Determine the number of 3s electrons in Na. 2The number of electrons in 4d subshell of Mo III 3. Use no variables other than k.

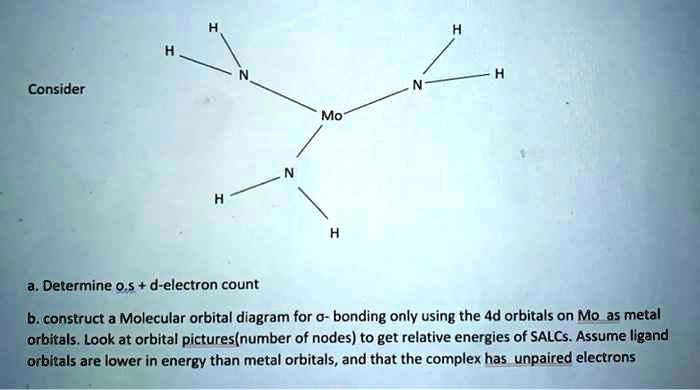
Solved Consider Mo Determine 0 D Electron Count B Construct A Molecular Orbital Diagram For Bonding Only Using The 4d Orbitals On Mo As Metal Orbitals Look At Orbital Pictures Number Of Nodes

Molybdenum Mo Electron Configuration And Orbital Diagram

This Atomic Structure Scientific Investigation Lab Activity Will Help Students Identify How Various Mo Scientific Investigation Lab Activities Atomic Structure
0 Comments